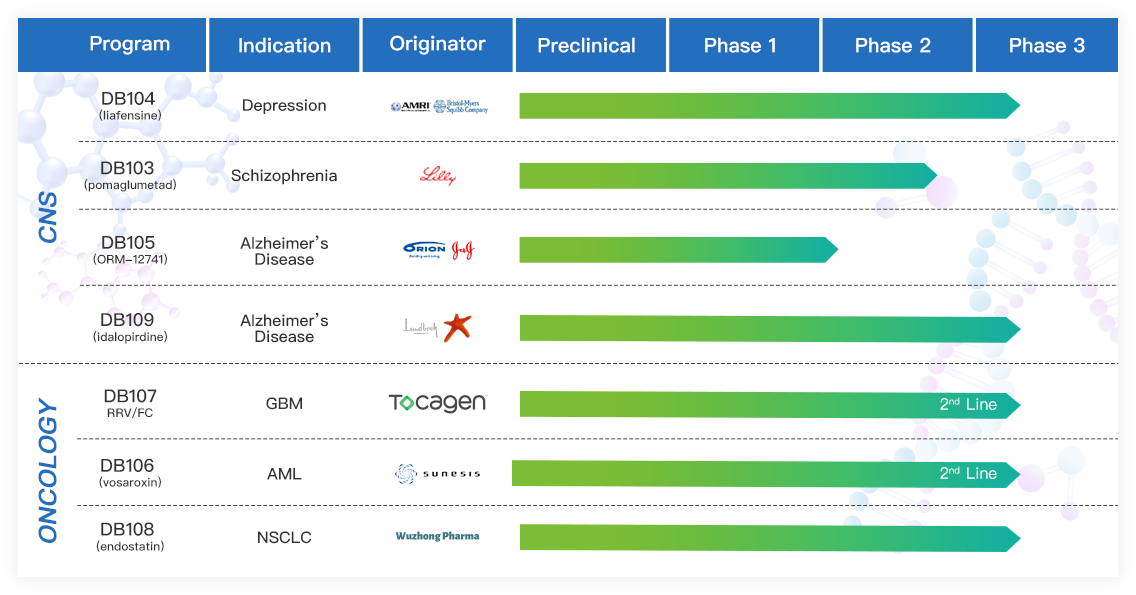
Rich Pipeline


Expanded access, also called compassionate use, enables patients with serious or immediately life-threatening diseases who do not meet the enrollment criteria for clinical trials in progress to gain access to investigational treatments. At this time, Denovo does not offer an expanded access program and does not accept expanded access requests. We believe that investigational drugs should be studied in patients as part of clinical trials designed to produce data on safety and efficacy that may be used to support approval of the product, thereby leading to its broader availability for patients in need of treatment. Denovo strongly encourages patients to speak with their treating physicians, and when possible, to participate in clinical trials. Denovo is aware that in rare cases patients with serious life-threatening diseases are unable to participate in clinical trials and may have exhausted all available therapies.
In these rare cases, Denovo may consider providing an investigational product outside of a clinical trial. However, we currently do not have an expanded access program that allows patients to have access to our investigational products prior to FDA approval. As authorized by the 21st Century Cures Act, Denovo may revise this expanded access policy at any time. Additionally, the posting of this policy by Denovo shall not serve as a guarantee of access to any specific investigational drug by any individual patient. In the event Denovo decides toconsider expanded access, we will evaluate and respond to each request that it receives on a case-by-case basis.
Reference information about our investigational drugs and ongoing clinical trials can be found on https://www.denovobiopharma.com and https://clinicaltrials.gov. If you have additional questions, please speak with your physician or contact us at info@denovobiopharma.com.